
X Cells
The Rolls Royce of Stem Cells
The Rolls Royce
of Stem Cells
After working in the field of regenerative medicine for over 25 years, I have seen many stem cell applications and numerous types of stem cells.

unique characteristics.
Stem cells can be autologous (from your own body) or allogeneic (from a donor). The most common types of autologous stem cells come from bone marrow or adipose tissue.
Due to the more complex and invasive nature of autologous stem cell use, many clinics and physicians prefer allogeneic sources. These clinics typically utilize allogeneic cells from perinatal tissues, including cord blood, umbilical tissue, Wharton's jelly, and amniotic fluid.
Additionally, there are products containing exosomes, which are small vesicles secreted by stem cells from these perinatal tissues that trigger a rejuvenating effect. We will delve into the topic of exosomes more extensively later.
over the years we found the clinical results of
perinatal tissue based stem cells to be less robust than stem cells from bone marrow and adipose tissue.
Until recently, we considered them to be the best option for off-the-shelf allogeneic stem cells. About a year ago, however, we discovered a new alternative that proved to be much more potent.
We refer to these as the X cells.
Derived from adipose or fat tissue donated by young, healthy, highly vetted individuals, X cells comprise approximately 10 different types of stem cells. We will later describe these stem cells and explain why their combination could be a superior treatment compared to other options. Among the primary stem cells present in X cells are the ADSCs, or adipose-derived stem cells.
After an extensive review of the research, I am persuaded that they may represent the best option for patients seeking regeneration in orthopedic, neurologic, and autoimmune conditions.
X cells have 5 characteristics that make them superior:

STRONG SURVIVAL
They are resilient and can survive in harsh environments, such as within damaged tissues where we need them most. X Cells live a minimum of 3 weeks where most other stem cell types used only survive 1-2 hours! Most clinics are using “perinatal stem cells” such as Wharton’s jelly, cord blood or other placental-derived sources. Most “Stem Cell” clinics out of the USA use culture-expanded perinatal stem cells.

Fast-acting
X cells differentiate four times faster than perinatal mesenchymal stem cells, or MSCs. This means they can start working faster to regenerate your body.

VASCULARIZATION
X cells are superior at producing endothelial cells at a much more rapid rate. That means more blood supply to the new cells and tissues so the body can bring in all the necessary oxygen and nutrients to create cartilage, nerve tissue, ligaments, and muscles.
BLOCK CANCER FORMATION
Muse cells contained in X cells inhibit cancer and teratoma formation, making them far superior than other options.

Immune tolerance
X cells have the potential to establish immune tolerance. “This is due to enhanced levels of immunomodulatory cytokines and increased VEGF-A and CD31 expression as well as reduced infiltration and proliferation of T lymphocytes, along with raised Treg expressions.
A Soup Of Regenerative Cells In X Cells
Both your bone marrow and fat contain a tremendous reservoir of regenerative nutrients. Your bone marrow is where all your stem cells are made. Like bone marrow, your adipose tissue contains many different types of stem cells. In fact, 1 out of every 100 cells in your fat is a stem cell!
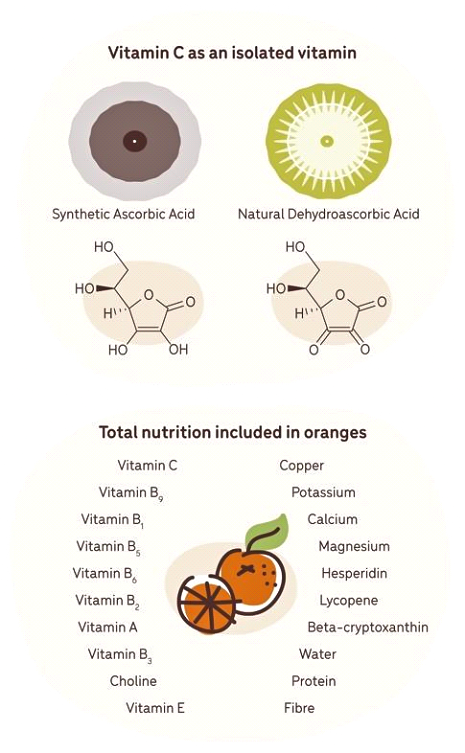
Consider a whole food like wheat bread, which provides far more nutrients compared to white bread that is devoid of vitamins and minerals and has a low nutrient value. Another example would be consuming synthetic forms of vitamin C versus natural sources such as oranges, amla, or gooseberries.
A study in 2007 compared the benefit of 150 mg of vitamin C from blood oranges versus 150 mg of synthetic vitamin C and found that the natural source provided a higher antioxidant effect. The researchers assumed it was due to the synergistic support of the various substances within the orange, such as polyphenols, bioflavonoids, rutin, catechins, anthocyanosides, and enzymes.
This proves what the hippies have been saying for years: Whole food sources of nutrients are more bioavailable and absorbable than isolated and synthetic sources. This is why eating foods that are dense in nutrients, like amla and other superfoods, can be a great way to prevent disease and provide vital nutrients that your brain and body need.
synthetic vitamin C vS.
natural sources:
The Entourage Effect

The notion that whole-food sources are more beneficial than their nutrients in isolation is not uncommon. The cannabis plant also largely depends on the presence of a spectrum of phytocannabinoids to create the synergy that results in its well known therapeutic effects.
In a study titled “The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No ‘Strain,’ No Gain,” the researchers conclude that because full-spectrum CBD (the second most prevalent active ingredient in cannabis) contains all cannabis plant compounds, it may have a more potent effect than pure CBD in an isolated form, resulting in greater health benefits. This is what researchers call the “entourage effect.”
In the same way, the body has provided us with reservoirs of regenerative “whole food” sources in our bone marrow and adipose tissue. In our fat, this reservoir contains fibroblasts, ADSCs, Muse stem cells, endothelial and hematopoietic progenitor stem cells, and supporting growth factors — a veritable soup of healing cells and nutrients. I call our bone marrow and adipose tissue the “whole food” of regenerative medicine.
Our clinic started using both fat and bone marrow in stem cell treatments in 2005, and I haven’t found anything that creates a regenerative outcome as powerful and durable. I’ve seen cases a decade or more post-treatment and patients are still pain-free and living their best life.
They assumed it was due to the synergistic support of the various substances within the orange such as polyphenols, bioflavonoids, rutin, and catechins and anthocyonides and enzymes. This proves what the hippies have been saying for years, wholes food sources of nutrients are more bioavailable and absorbable than isolated and synthetic sources.
In the same way, the body has provided us reservoirs of regenerative “whole food” sources in our bone marrow and adipose tissue. In our fat this reservoir contains fibroblasts, ADSC’s, MUSE stem cells, endothelial and hematopoietic progenitor stem cells, Supporting growth factors and fibroblasts.
A soup of healing cells and nutrients.
I call this the “whole food” or regenerative medicine.
Our clinic started using both fat and bone marrow in 2005 and I haven’t found anything that creates a regenerative outcome as powerful and durable as they do. I’ve seen cases a decade or more post-treatment and they are still pain free living their best life.
X cells are a complex mix containing ADSCs as well as hundreds of stem cell types, growth factors, and supporting cells, including:
Mesenchymal Stem Cells (MSCs): MSCs typically make up 6-12% of X Cells.
Hematopoietic (HSCs) and Endothelial progenitor cells (EPCs): These cells contribute to blood vessel formation. ADSC exosomes contain adipokines that increase angiogenesis and adipogenesis while decreasing inflammation [9]. Note: Endothelial cells are removed from X cells as they contain ACE-2 receptors and could be a vector for transmission of spike proteins.
Supporting Stem Cells: These include fibroblasts, endothelial cells, pericytes, and immune cells. They play a crucial role in the overall function of X cells by providing structural support, secreting growth factors, and modulating the immune response.
Multilineage-differentiating stress-enduring (Muse) cells: Muse cells are a stress-tolerant population of stem cells found in both bone marrow and adipose tissue. Muse stem cells are pluripotent, meaning they can turn into any cell line. They can also endure hostile environments, supporting their survival in damaged or injured tissues.[11] They support both ectodermal tissues (brain, neurological) and endodermal tissues (lung, thyroid, and pancreas). Furthermore, Muse cells have inherent immunomodulatory properties which support calming down inflammation and autoimmune conditions as well as contribute to tissue regeneration and repair.[12]
Preadipocytes: Preadipocytes are the most abundant cell type in the fraction from adipose tissue. Recent evidence suggests that this cell, also described as a supra-⦁ adventitial adipose stromal cell or dedifferentiated adipose cell, shares many of the same phenotypic markers and characteristics of MSCs, implicating its involvement in regeneration.[13] It is also the precursor to the mature adipocyte
Endothelial progenitor cells: EPCs have the capacity to induce angiogenesis through the release of growth factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1).[14]

There are now hundreds of companies producing various culture-expanded stem cells which are grown in a lab to make duplicates of a particular cell.
They are typically from placental origin or perinatal tissue. My perspective is that it’s like white bread, devoid of the gamut of supporting and synergistic cell lines that provide healing.
They lack the majesty in our bone and fat put there by creation itself.
Some companies using these “white bread” stem cells have started using gene therapy to activate cells to become certain cell types. It certainly sounds sexy and commands a higher price point.
But is this kind of gene manipulation really necessary when nature has already provided our bodies with everything they need to heal and regenerate?
A Look at Stem Cell Isolation:
ADSC Isolation vs SVF
Adipose-derived stem cells are isolated from the adipose tissue using the “whole food” of the fat, which would include the fraction.
This “whole food” of adipose tissue is called the stromal vascular fraction, or SVF.
One study found an advantage of SVF over ADSCs in two fundamental areas.
One is that the diversity of cells, or the heterogeneous cellular composition of SVF, may be responsible for the better therapeutic outcomes observed.
Secondly, SVF is obtained with minimal contact with reagents, making it comparatively safer.


X cells are processed entirely with gravity alone, using zero reagents or chemicals.
Clearly, with bone marrow and adipose, nature has packaged the proper assortment of regenerative properties perfectly, and things only become less effective when we manipulate and change them, often for the purpose of creating intellectual property in order to patent them for financial gain.
It’s a regenerative medicine “gold rush” right now, and Wall Street and big financial institutions want a piece of the pie.
Much like Big Pharma, companies are filing patents and commercializing something that is much more effective the way nature provided for us to repair our bodies.
Hair, Skin & Sexual Rejuvenation
X cells have an enhanced ability to produce both endothelial cells and regenerate nerves, which makes them an excellent choice for treating hair loss, sexual rejuvenation (O-Shot and P-Shot), and even skin rejuvenation.
In one study, researchers looked at the isolation of ADSCs versus using ADSCs along with the fraction, or SVF. They concluded that “SVF treatment showed superior statistically significant results compared to ADSC treatment alone, especially in the smooth-muscle-to-collagen ratio and in endothelial cell content.
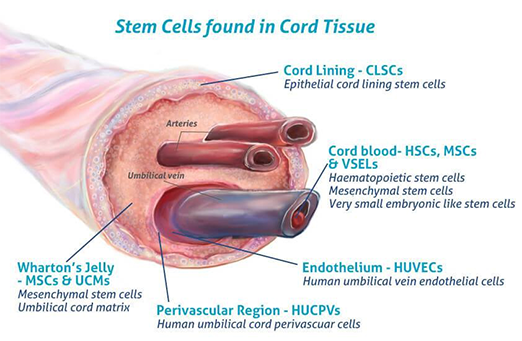
COMPARING UMBILICAL AND PERINATAL STEM CELLS TO X CELLS
Poor Survival
Perinatal tissues have poor survival and may only last 1 hour. Studies have shown that allogeneic MSCs from perinatal tissues face challenges in long-term survival and immunogenicity after transplantation, impacting their therapeutic efficacy.
That’s a huge difference compared to the three weeks X cells can survive. In one study, X cells were still present and alive 10 weeks later!
There is an abundance of literature that supports the vastly superior survival rate and regenerative potential of ADSCs and X cells compared to perinatal-sourced stem cells, including those derived from amniotic fluid, the umbilical cord, and placenta. This evidence is crucial for understanding the optimal source of stem cells for regenerative medicine applications.
In a nutshell, X cells survive longer, so they work longer and continue the regeneration process for weeks after implantation. Other stem cell sources, by contrast, die quickly and are no longer replicating, no longer differentiating, and no longer transmitting extracellular vesicles (EVs) like exosomes.
Perinatal stem cells have been shown to be sensitive to harsh environments once transplanted. The study “Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium” shows that survival, which requires “engraftment” (or the ability of these stem cells to actually work) is poor with perinatal stem cells.
POOR NICHE FORMATION
Perinatal stem cells lack niche and trophic support once introduced into the body. Niche and trophic support are created through complex support from stem cells, producing growth factors.
Although these stem cells do produce growth factors, they don’t do well once transplanted into the body due to the poor signaling quality of perinatal stem cells. They must rely on the growth factors contained within the dose used during the treatment versus having the ability to be their own growth factor factory once they are transplanted.
What is niche support, and why does it matter for stem cell survival?
Once stem cells are transplanted into a joint or another area of the body, they will survive and thrive, they will die, or they will become senescent (a permanent state of sleep). A niche, or the environment the cells will be subjected to, can be harsh or hospitable. The niche greatly impacts the course these cells will take. As mentioned earlier, the stem cell lines contained in X cells are durable and resist becoming stressed even when placed into a harsh environment. They are simply better than any other stem cell lines studied. Why is this the case? This is where we need to look at the niche.
Many chronic injuries that stem cell therapy treats require these cells to endure harsh environments. What makes the environment harsh is the level of inflammation, which is also associated with oxidation. Research has shown that X cells have high chances of survival in high-oxidation environments.[19]
In addition to being resilient to harsh environments, X cells have the ability to rapidly create a more hospitable niche once they are placed there. They do that by bringing in endothelial cells that create new blood supply, which then brings in nutrients like amino acids and oxygen. Another ability X cells have is producing substances called growth factors which further support the expansion and differentiation process.
In the study, “Engineering of an angiogenic niche by perfusion culture of adipose-derived stromal vascular fraction cells,” the researchers conclude, “The SVF leads to the engineering of a specialized milieu, here defined as an angiogenic niche. This supports the multicellular interactions in angiogenesis, which supports an increased grafted cell survival in regenerative medicine.”[20] The idea is that the collection of this “whole food” mix of cells allows for a rapid development of blood supply to begin providing nutrients to the stem cells and new tissues they are creating.
IMMUNE REJECTION
Perinatal stem cells have a high immunological rejection rate, which essentially means your immune system kills them. This is why they cause more pain and inflammation than X cells. This immune rejection is due to a mismatch of the human leukocyte antigen (HLA), and scientists suggest caution. It’s very important to perform an HLA assessment in off-the-shelf allogeneic mesenchymal stem cell-based therapies.[21] I don’t know even one company doing this that sells these products to doctors!
Similarly, all “cross sex” umbilical donor cells have engraftment issues in which engraftment, if it happens at all, is dubious. In order to prevent graft failure, the perinatal tissue would need to be processed so the MSCs would be isolated and expanded and produce only one generation in a bioreactor.
Using just one generation would allow for limited mutations, if any, and you would not have the MHC-2 expression that causes HLA-matching or microchimerism negatives (defined by the presence of circulating cells, bi-directionally transferred from one genetically distinct individual to another).[22] Most companies do several generations of expansion for higher yields!
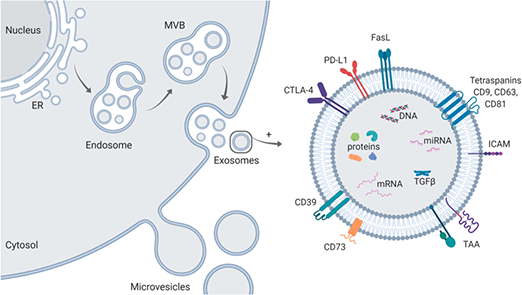
EXOSOMES - Let’s circle back to exosomes. Stem cells not only migrate to damaged tissue and differentiate into various cell lines to replace the damaged cells, they also attach in locations adjacent to damaged cells and release tiny vesicles called exosomes. Exosomes are tiny packages of RNA. RNA carries information, and these little vesicles carry information from the stem cell.
This will influence the damaged cells as well as healthy cells in the area to go into a regenerative phase and begin to rebuild damaged tissue with new, fresh cells and tissue.
Ultimately, it is your own cells and tissue that do the repair after the RNA in the exosomes showers the injured or weakened sites in the body.
Exosomes that are released by the stem cells provide the primary influence for regeneration. Both perinatal and ADSC stem cells produce an average of 17 million exosomes per hour. Let’s get into some deep math to prove my point as to the benefits of a longer-lived stem cell line.
An X cell producing 17 million exosomes per cell per hour (which is the average, per studies) multiplied by 2 million X cells is 34 trillion per hour, which would continue to be produced for 2–4 weeks.
When we compare this to a typical perinatal stem cell producing 17 million exosomes over 12 hours (12 hours divided by the 504 hours in 3 weeks), it is only 2.5% of what an X cell would create over 3 weeks.
That’s 4,000% more regenerative ability!
X Cells Clinically
X cells have been FDA-approved for various treatments, including the treatment of perianal fistulizing with Crohn’s disease administered via IV infusion.[23] There have been 5,340 patients treated under an FDA-approved multisite clinical trial for osteoarthritis of the knee utilizing X cells.
There have been over 200 clinical stem cell trials utilizing X cells, more than any other stem cell source to date. X cells are natural, not expanded, and processed without DMSO or enzymes. And to date, each audit by the FDA has resulted in a perfect review. Advanced Rejuvenation is the first clinic to use X cells for inner ear regeneration, and we plan to begin clinical trials soon based on positive results.
X Cells For Brain & Nerves
Researchers have found X cells to show superior potential for promoting nerve regeneration and remyelination of nerves in cases of MS and neuropathy. When administered to damaged nerve fibers, X cells can incorporate themselves into the affected area, differentiate into oligodendrocytes (the cells responsible for producing myelin), and initiate the rebuilding of the myelin sheath.
X cells promote adult neurogenesis in the brains of Alzheimer’s patients. X cells are exquisitely sensitive to their environment with regard to neurogenic marker expression or receiving chemical signaling to become various types of nerve cells. Research has shown that X cells carry further significant advantages, as they can differentiate toward neurogenic lineage.
BRAIN PROTOCOL



At Advanced Rejuvenation, we see many neurological cases and utilize a powerful protocol called Red Blue O2. This is a synergistic treatment using photobiomodulation (red light therapy), methylene blue, and other photodynamic substances along with oxygen therapies such hyperbaric oxygen (HBOT), CVAC, and cranial therapy, which aims to improve oxygen within the brain. We use a special process along with the administration of X cells in a way that allows them to access the brain by crossing the blood-brain barrier. For more on Red Blue O2, see the book Methylene Blue: Magic Bullet on Amazon.
If you are a patient interested in finding out if X cells might help you with a health issue, or you’re a healthcare provider and you’d like to find out if you might qualify to be one of a limited number of clinics providing X cell technology, please contact us via the online form below.
Dr. John Lieurance, ND, DC
ADVANCED REJUVENATION

INTERESTED IN X CELLS?
If you are a patient interested in discovering whether X Cells could assist you with a health issue, or if you're a healthcare provider looking to determine your eligibility, please complete our intake form below to begin your qualification process.
Advanced Rejuvenation
1014 North East Avenue, Sarasota, Fl, 34237
+1 (941) 330 - 8553
Copyright Advanced Rejuvenation 2024
References
⦁ Li, M., Luo, X., Lv, X. et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther 7, 160 (2016). https://doi.org/10.1186/s13287-016-0420-2
⦁ Huang, Hui-Kuanga–d; Hsueh, Kuang-Kaie; Liao, Yu-Tingb,f; Wu, Szu-Hsiena,f,g; Chou, Po-Hsinb; Yeh, Shih-Hane; Wang, Jung-Pana,b. Multilineage differentiation potential in the infant adipose- and umbilical cord-derived mesenchymal stem cells. Journal of the Chinese Medical Association 86(12):p 1083-1095, December 2023. | DOI: 10.1097/JCMA.0000000000000990
⦁ Steiner D, Mutschall H, Winkler S, Horch RE, Arkudas A. The Adipose-Derived Stem Cell and Endothelial Cell Coculture System-Role of Growth Factors? Cells. 2021 Aug 13;10(8):2074. doi: 10.3390/cells10082074. PMID: 34440843; PMCID: PMC8394058.
⦁ Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014 Apr 1;23(7):717-28. doi: 10.1089/scd.2013.0473. Epub 2014 Jan 17. PMID: 24256547.
⦁ Chen J, Wang Y, Hu H, Xiong Y, Wang S, Yang J. Adipose-derived cellular therapies prolong graft survival in an allogenic hind limb transplantation model. Stem Cell Res Ther. 2021 Jan 29;12(1):94. doi: 10.1186/s13287-021-02162-7. PMID: 33514430; PMCID: PMC7847016.
⦁ Prantl L, Eigenberger A, Brix E, Kempa S, Baringer M, Felthaus O. Adipose Tissue-Derived Stem Cell Yield Depends on Isolation Protocol and Cell Counting Method. Cells. 2021 May 5;10(5):1113. doi: 10.3390/cells10051113. PMID: 34063138; PMCID: PMC8148142.
⦁ Almog Uziel, Anat Gelfand, Keren Amsalem, Paula Berman, Gil M. Lewitus, David Meiri, and Dan Y. Lewitus. Full-Spectrum Cannabis Extract Microdepots Support Controlled Release of Multiple Phytocannabinoids for Extended Therapeutic Effect. ACS Applied Materials & Interfaces, 2020 12 (21), 23707-23716 DOI: 10.1021/acsami.0c04435
⦁ Russo EB. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No "Strain," No Gain. Front Plant Sci. 2019 Jan 9;9:1969. doi: 10.3389/fpls.2018.01969. PMID: 30687364; PMCID: PMC6334252.
⦁ Cai, Y., Li, J., Jia, C. et al. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther 11, 312 (2020). https://doi.org/10.1186/s13287-020-01831-3
⦁ James C. Brown, Adam J. Katz. Stem Cells Derived From Fat. Principles of Regenerative Medicine (Third Edition), 2019
⦁ Alanazi RF, Alhwity BS, Almahlawi RM, Alatawi BD, Albalawi SA, Albalawi RA, Albalawi AA, Abdel-Maksoud MS, Elsherbiny N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells. 2023 Jun 21;12(13):1676. doi: 10.3390/cells12131676. PMID: 37443710; PMCID: PMC10340735.
⦁ Fisch SC, Gimeno ML, Phan JD, Simerman AA, Dumesic DA, Perone MJ, Chazenbalk GD. Pluripotent nontumorigenic multilineage differentiating stress enduring cells (Muse cells): a seven-year retrospective. Stem Cell Res Ther. 2017 Oct 18;8(1):227. doi: 10.1186/s13287-017-0674-3. PMID: 29041955; PMCID: PMC5646122.
⦁ James Guo, Andrew Nguyen, Derek A. Banyard, Darya Fadavi, Jason D. Toranto, Garrett A. Wirth, Keyianoosh Z. Paydar, Gregory R.D. Evans, Alan D. Widgerow,
Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. Journal of Plastic, Reconstructive & Aesthetic Surgery, Volume 69, Issue 2, 2016, Pages 180-188, ISSN 1748-6815. https://www.sciencedirect.com/science/article/pii/S1748681515004982
⦁ Bora, P., Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8, 145 (2017). https://doi.org/10.1186/s13287-017-0598-yBora, P., Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8, 145 (2017). https://doi.org/10.1186/s13287-017-0598-y
⦁ Li, M., Luo, X., Lv, X. et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther 7, 160 (2016). https://doi.org/10.1186/s13287-016-0420-2
⦁ Burova E, Borodkina A, Shatrova A, Nikolsky N. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxid Med Cell Longev. 2013;2013:474931. doi: 10.1155/2013/474931. Epub 2013 Aug 25. PMID: 24062878; PMCID: PMC3767075.
⦁ Ibid.
⦁ Cerino, G., Gaudiello, E., Muraro, M.G. et al. Engineering of an angiogenic niche by perfusion culture of adipose-derived stromal vascular fraction cells. Sci Rep 7, 14252 (2017). https://doi.org/10.1038/s41598-017-13882-3
⦁ Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The Importance of HLA Assessment in "Off-the-Shelf" Allogeneic Mesenchymal Stem Cells Based-Therapies. Int J Mol Sci. 2019 Nov 13;20(22):5680. doi: 10.3390/ijms20225680. PMID: 31766164; PMCID: PMC6888380.
⦁ Drozina G, Kohoutek J, Jabrane-Ferrat N, Peterlin BM. Expression of MHC II genes. Curr Top Microbiol Immunol. 2005;290:147-70. doi: 10.1007/3-540-26363-2_7. PMID: 16480042.
⦁ Bislenghi G, Wolthuis A, Van Assche G, Vermeire S, Ferrante M, D'Hoore A. Cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn's disease. Expert Opin Biol Ther. 2019 Jul;19(7):607-616. doi: 10.1080/14712598.2019.1623876. Epub 2019 Jun 3. PMID: 31121104.
⦁ Hedayatpour A, Ragerdi I, Pasbakhsh P, Kafami L, Atlasi N, Pirhajati Mahabadi V, Ghasemi S, Reza M. Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis. Cell J. 2013 Summer;15(2):142-51. Epub 2013 Jul 2. PMID: 23862116; PMCID: PMC3712775.
⦁ Yan, Yufang1; Ma, Tuo1; Gong, Kai1; Ao, Qiang2; Zhang, Xiufang1; Gong, Yandao1,. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer's disease mice. Neural Regeneration Research 9(8):p 798-805, April 15, 2014. | DOI: 10.4103/1673-5374.131596
⦁ Junmin Lee, Amr A. Abdeen, Xin Tang, Taher A. Saif, Kristopher A. Kilian. Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomaterialia, Volume 42, 2016, Pages 46-55, ISSN 1742-7061. https://www.sciencedirect.com/science/article/pii/S1742706116303142
⦁ Pietro Gentile, Augusto Orlandi, Maria Giovanna Scioli, Camilla Di Pasquali, Ilaria Bocchini, Valerio Cervelli, Concise Review: Adipose-Derived Stromal Vascular Fraction Cells and Platelet-Rich Plasma: Basic and Clinical Implications for Tissue Engineering Therapies in Regenerative Surgery, Stem Cells. Translational Medicine, Volume 1, Issue 3, March 2012, Pages 230–236, https://doi.org/10.5966/sctm.2011-0054
⦁ Adamiak M, Moore JB, Zhao J, et al. Downregulation of Heme Oxygenase 1 (HO-1) Activity in Hematopoietic Cells Enhances Their Engraftment after Transplantation. Cell Transplantation. 2016;25(7):1265-1276. doi:10.3727/096368915X688957
⦁ Kristine M Safford, Kevin C Hicok, Shawn D Safford, Yuan-Di C Halvorsen, William O Wilkison, Jeffrey M Gimble, Henry E Rice. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochemical and Biophysical Research Communications, Volume 294, Issue 2, 2002, Pages 371-379, ISSN 0006-291X.
https://www.sciencedirect.com/science/article/pii/S0006291X02004692